Team Rewires a Behavioral Circuit in the Worm Using Hydra Parts

WOODS HOLE, Mass. — For two people to communicate in a loud, crowded room, they need to be standing side by side. The same is often true for neurons in the brain. But the same way a cell phone allows two people to communicate clearly across the room, new research at the Marine Biological Laboratory (MBL) opened up a new channel of communication in the worm C. elegans brain.
The research, published in Nature Communications, highlights the development of HySyn, a system designed to synthetically reconnect neural circuits using neuropeptides from Hydra, a small, freshwater organism. (Neuropeptides modulate the activity of neurotransmitters to increase or decrease the strength of impulses between neurons.)
For the first time, the researchers created genetic lines of mutant C. elegans that expressed neuropeptides from the Hydra brain, creating an artificial synapse that rewired a behavioral circuit in the worm. Because none of the other synapses in the brain, besides those fitted with the hydra receptor and neuropeptide, could hear the “command,” it was like giving them a cell phone so they could communicate.
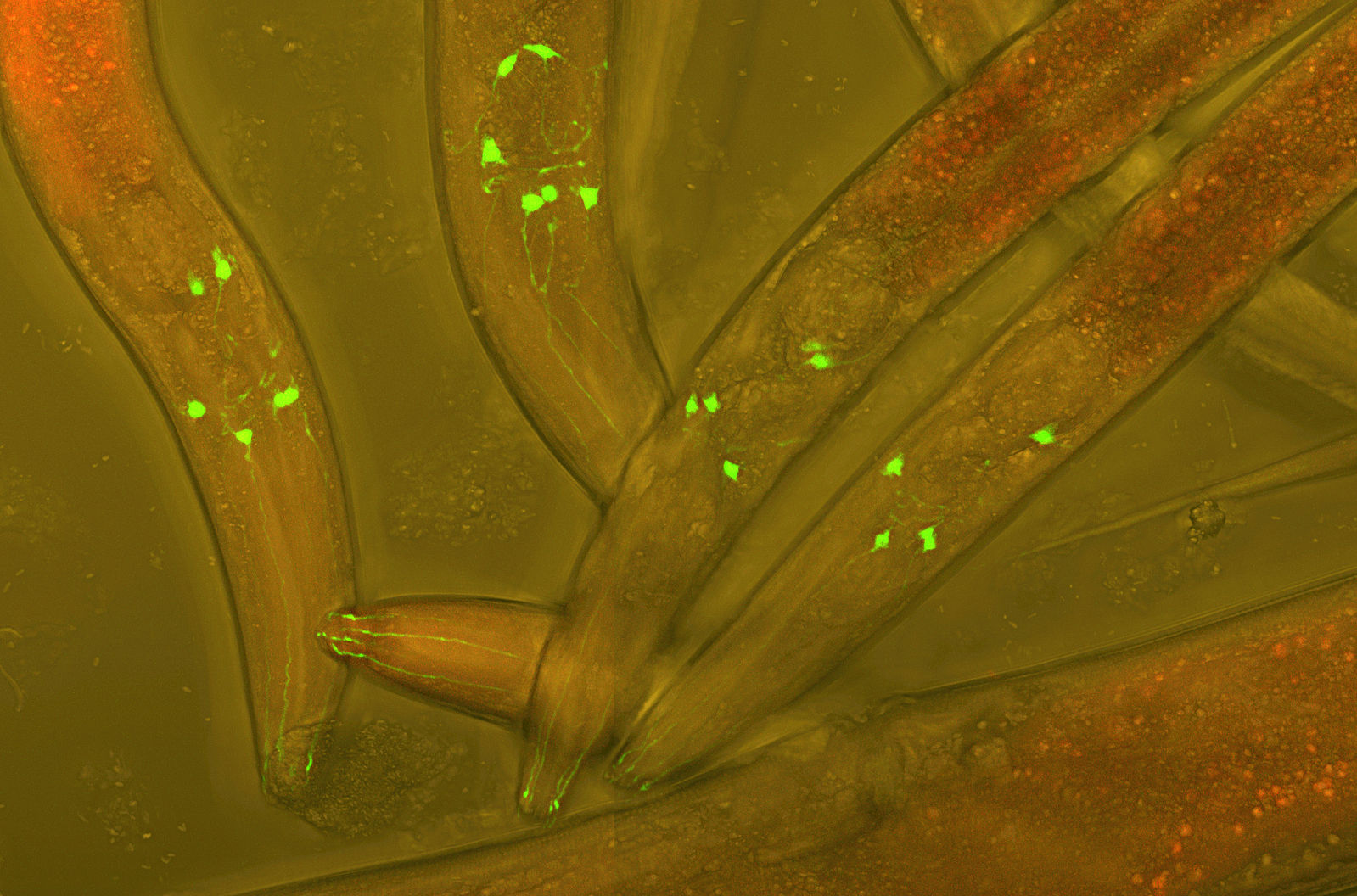 C. elegans worms with green fluorescent protein (GFP) inserted into their neurons to visualize neural development. Photo Credit: Heiti Paves, Wikimedia Commons.
C. elegans worms with green fluorescent protein (GFP) inserted into their neurons to visualize neural development. Photo Credit: Heiti Paves, Wikimedia Commons.“These neuromodulatory peptides let you communicate at a distance,” said MBL Fellow Daniel Colón-Ramos of Yale University School of Medicine. “It gives you more flexibility as a researcher to manipulate neurons that are not adjacent to each other.” Colón-Ramos, senior author on the paper, was postdoctoral advisor for the paper’s first author, former MBL Grass Fellow Josh Hawk. The work and analysis was performed at the MBL and at Yale University in Colón-Ramos’s lab.
The researchers used a mutant line of C. elegans that was missing the neural connection that controlled specific behavior — the behavior that told them that they were full and needed to stop searching for food. By taking genes that encode a neuropeptide and its receptor from Hydra and putting them into the C. elegans worm, researchers were able to restore the neural circuit that controls this behavior. They created two separate genetic lines—one that contained the neuropeptide and one that contained the receptor. The offspring of the pair contained the full neural peptide pathway. But, according to Hawk, it’s just one possible pathway to focus on.
“There are hundreds of neural peptides in Hydra, each of which could be a different channel of communication,” said Hawk. “To me, that’s the most exciting thing. This should open up a whole area that no one has ever explored before.”
Hawk called this study a “proof of principle” for the HySyn tool. Unlike most organisms, Hydra don’t have a classic neurotransmitter system in the brain. Instead, they rely completely on a net of neuropeptides. Each of these Hydra neuropeptides has the opportunity for a unique line of communication. Hawk focused on a particular neuropeptide that creates a slow-building signal, like the slow-building sensation of fullness as you eat a meal. Connecting different neurons with this neuropeptide could create a slowly rising pain response or strengthen a new memory.
There are many different types of communication in the brain. Some are fast, on the order of milliseconds. But others are slower. That’s what Hawk was focusing on, the slow neuromodulatory response that told the C. elegans: “I’m full, don’t go seek out new food.” The researchers created an artificial, neuromodulatory synapse that told the C. elegans it was time to relax.
“Usually, we can break things in science. That’s very powerful. When we break things we can see what they are necessary for, which also tells us a little about how they work. But to really understand how they work, you want to know if you can rebuild them—fix them—after they are broken. And that is very hard to do,” said Colón-Ramos. “That [Hawk] could rebuild the connections between cells was very exciting and innovative.”
Hawk wasn’t just putting back missing components in the C. elegans brain; he was putting in entirely new components from Hydra -- fixing what was broken with a different set of parts. It showed the researchers that it wasn’t necessarily the identity of the parts themselves, but the communication and modulation between the synapses that regulated the behavior.
Hawk said he hopes future research focuses on knocking out lines of communication in the brain and rebuilding them in a different way with HySyn. “This is the beginning of a set of tools, and as those tools are expanded, it gives us a real ability to tweak connections in the brain in a variety of permutations,” he said.
 Hydra are freshwater organisms, only a few millimeters in length. Here, muscle fibers are in sepia and the nerve net is in blue/green.
Hydra are freshwater organisms, only a few millimeters in length. Here, muscle fibers are in sepia and the nerve net is in blue/green.Credit: John Szymanski, Current Biology
“There’s a lot of diversity of synaptic connection in any animal’s brain. Being able to pick and choose what to put in another organism will help us untangle and understand how and why brains do what they do,” said Hawk.
The researchers are confident that HySyn will work in a variety of organisms. They tested it in vertebrate cells as well as in C. elegans.
Hawk added that he hopes researchers will focus on learning about the strength of connections within the brain. “In theory, can you tear down the worm brain and then rebuild it and find out what is lost when you rebuild it certain ways. It’s what we did as kids: If you want to know how something works, you break it down and rebuild it. But we need more tools to do that.” HySin represents a contribution to that toolset.
In addition to his time as a Grass Fellow in 2019, Hawk was faculty member for the MBL’s Neural Systems and Behavior (NS&B) course from 2015-2019.
“Josh was able to leverage knowledge from the MBL and the people he met and put all that knowledge together, with his own interests in neuroscience, and make a significant contribution. This is what programs like the Grass Fellowship and MBL help catalyze in science,” said Colón-Ramos.
“It’s like an emblematic MBL project,” Colon-Ramos said. “High risk, high reward. Lots of fun and bringing in a lot of knowledge from the MBL community.”
Citation:
Hawk, J.D., Wisdom, E.M., Sengupta, T. et al. (2021) A genetically encoded tool for reconstituting synthetic modulatory neurotransmission and reconnect neural circuits in vivo. Nature Communications, DOI: 10.1038/s41467-021-24690-9
Homepage photo:
The C. elegans worm is a common model organism. Photo Credit: Zeynep F. Altun, Wikimedia Commons
