That crab has rhythm: Exploring controls on digestion in Neural Systems & Behavior

When it comes to explaining crab digestion, three-letter acronyms abound. Luckily, Neural Systems and Behavior course instructor David Schulz of the University of Missouri comes to Woods Hole to translate. Schulz aims to decipher not only the lexicon of crustacean feeding, but also how gene expression affects the neural network processes responsible.
Schulz studies the stomatogastric ganglion (STG), a collection of neurons generating electrical signals that control the three teeth located in the crab’s stomach, producing a chewing motion as well as the filter that controls food entering the gut for digestion. Because these actions are rhythmic (“patterned”) processes, the STG is one example of a central pattern generator (CPG).
 Neural Systems and Behavior course instructor, David Schulz of the University of Missouri.
Neural Systems and Behavior course instructor, David Schulz of the University of Missouri.The question of how the nervous system produces rhythmic output like breathing, walking, and in this case chomping, still remains unanswered in neuroscience. Since CPGs are present in more complex organisms, investigating the crab STG can inform human conditions related to patterned behaviors (e.g., spinal cord injury).
Consisting of only about 25 cells, the crab STG is known for its simplicity. In addition to being easily manipulated using drugs or by altering electrical signals between neurons, this system can also remain isolated in a dish for days during experiments.
While scientists were traditionally interested in measuring the electrical currents coursing through STG cells to generate behavior, the field of biology is now trending toward genetic analyses. Schulz does not intend for the crab to be left in the dust, and has begun probing his subjects on a molecular level.
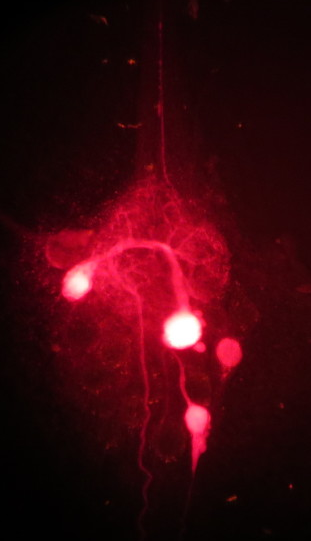 Crab stomatogastric ganglion (STG) with tracer-filled cells. Credit: Neural Systems and Behavior faculty, 2015.
Crab stomatogastric ganglion (STG) with tracer-filled cells. Credit: Neural Systems and Behavior faculty, 2015.In particular, he is interested in the genes encoding the proteins that determine which electrically charged molecules (“ions”) enter the neuron and when—creating electrical currents that relay messages both inside and between neurons. Essentially, different levels of protein expression result in varying rhythmic behaviors. This means that, although the STG has very stereotyped actions (e.g. chewing), these simple patterns can occur at different rates and through nuanced motions depending on the size and consistency of the food.
Yet, what makes the STG unique, Schulz says, is that these neurons are incredibly robust. The behaviors they produce usually remain unchanged despite electrical and molecular interventions that impact protein expression levels. Since animals with robust CPGs, like the crab, can more easily adapt to increases in temperatures, Schulz thinks they may help scientists understand how organisms adapt to shifts in environment and even climate change. And that thought gives students quite a lot to chew on.