The Fantastic Voyage Within the Cell: Liam Holt's Research on Cytoplasmic Crowding

Within one cell, a tiny world exists, imperceptible to our clunky human senses without magnification. Molecules bounce around within a squishy cytoplasm, the substance that fills cells. Some of these molecules collide and interact to produce products for the cell.
But the molecules in a cell don’t behave the way objects do in our macro world. They have so little mass that their movements are largely driven by heat and the dynamic environment in which they exist.
Even stranger, the substance these molecules move in, cytoplasm, has unusual properties. It’s what is known as a viscoelastic material, meaning if you push on it quickly, it will seem solid, but if you press on it slowly, it will behave like a viscous liquid — think molasses.
“This research is a fantastic voyage within the cell — it gives us a sense of what it’s like to be a molecule traveling in the cytoplasm,” says Liam Holt, scientist at the New York University School of Medicine and 2018 Whitman Center Fellow at MBL. “Our intuition in this area is limited because these events happen at such a small scale. Tiny, nanoscale objects see this world in a very different way.”
It’s the exploration of this substance and how the molecules in it behave, called rheology, that is Holt's specialty. Holt and his colleagues wanted to know at what point molecules are close enough together to favor important interactions that drive the cell’s metabolism. At a certain point, molecules in the cell become too crowded, reaching what is known as a jamming transition, a phenomenon much like a traffic jam —molecules become stuck and cannot reach potential partners.
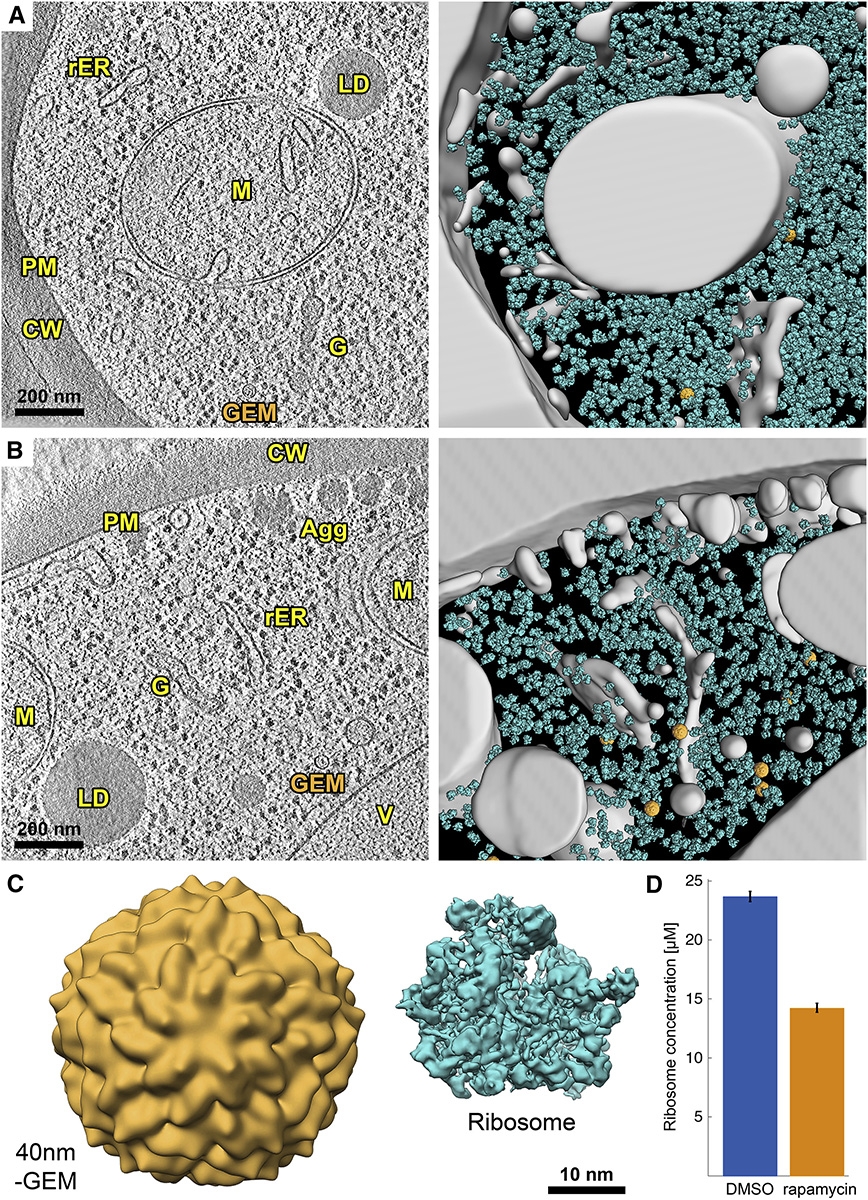
Holt uses a species of yeast, S. cerevisiae, a model organism for studying cellular processes, to measure crowding within the cell. But Holt and his colleagues faced a challenge: they didn’t have an easy way to measure how crowded the cytoplasm was. They needed a nanoscale ruler – something of known size that could be used to measure organelles and molecules within the cell.
Their simple, elegant innovation, called Genetically Encoded Multimeric nanoparticles, or GEMs for short, helped solve their problem. Each GEM measures 41 nanometers across. Genes that code for GEMs are injected into cells so they can produce and send them into the cytoplasm. Fluorescent proteins are attached to the GEMs so they glow brightly and are traceable, like small, colored jewels bouncing around among a cell’s organelles.
Tracking the GEMs’ movement, or lack thereof, Holt and colleagues found that ribosomes, protein factories suspended in the cytoplasm, are likely a main contributor to cytoplasmic crowding. After inhibiting the gene that tunes ribosome concentration, called TORC1, they noticed a dramatic decrease in ribosomes, yet an increase in the diffusion of other particles. This indicates that when fewer ribosomes are present, other particles can move more freely, and controlling ribosome concentration may control molecular interactions and the cell’s overall metabolism.
During his Whitman Center Fellowship this summer, Holt is collaborating with MBL scientists who specialize in moss, fission yeast, and other organisms, measuring molecular crowding and looking at its control through an evolutionary lens. Understanding how and why organisms control crowding may even lead to insight on the metabolism of disease cells, such as cancer.
“Our ultimate goal is to look at a much wider range of the tree of life and get an idea of how much variation there is,” says Holt. “We have the opportunity to look at how this function evolved.”
GEMs are minuscule but not insignificant — they’re making a major impact on cell research. The ability to vividly view and measure the nanoscale environment of a cell with the help of electron microscopy technology and GEMs introduces scientists to a whole new world, and one which they are only beginning to explore, pushing scientific frontiers at the tiniest scale.
Holt and colleagues recently published their results:
M. Delarue et al (2018) mTORC1 Controls Phase Separation and the Biophysical Properties of the Cytoplasm by Tuning Crowding. Cell, doi: 10.1016/j.cell.2018.05.042