Turning On the Switch for Plasticity in the Human Brain

The most powerful substance in the human brain for neuronal communication is glutamate. It is by far the most abundant, and it’s implicated in all kinds of operations.
Among the most amazing is the slow restructuring of neural networks due to learning and memory acquisition, a process called synaptic plasticity. Glutamate is also of deep clinical interest: After stroke or brain injury and in neurodegenerative disease, glutamate can accumulate to toxic levels outside of neurons and damage or kill them.
Shigeki Watanabe of Johns Hopkins University School of Medicine, a familiar face at the MBL as a faculty member and researcher, is hot on the trail of describing how glutamate signaling works in the brain to enable neuronal communication. In a paper last fall, Watanabe (along with several MBL Neurobiology course students) described how glutamate is released from neural synapses after the neuron fires. And today, Watanabe published a follow-up study in Nature Communications.
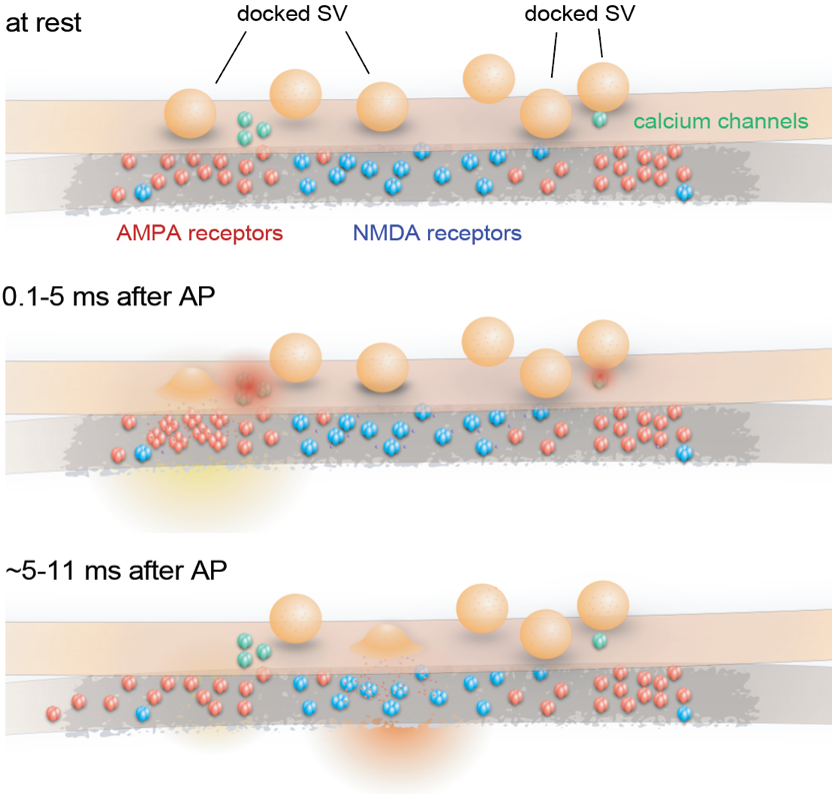 Schematic of proposed synaptic organization and events. Synchronous fusion begins within hundreds of microseconds near the AMPA receptors. Released glutamate activates AMPA receptors, which in turn depolarizes the membrane. Between 5 and 11 ms, residual calcium triggers asynchronous fusion, preferentially toward the center of the active zone and across from the NMDA receptors, favoring the NMDA receptor activation. This organization allows the maximal depolarization of the postsynaptic membrane and efficient activation of NMDA receptors – the switch for the plasticity. Credit: Shuo Li et al., Nature Comm., 2021
Schematic of proposed synaptic organization and events. Synchronous fusion begins within hundreds of microseconds near the AMPA receptors. Released glutamate activates AMPA receptors, which in turn depolarizes the membrane. Between 5 and 11 ms, residual calcium triggers asynchronous fusion, preferentially toward the center of the active zone and across from the NMDA receptors, favoring the NMDA receptor activation. This organization allows the maximal depolarization of the postsynaptic membrane and efficient activation of NMDA receptors – the switch for the plasticity. Credit: Shuo Li et al., Nature Comm., 2021“With this paper, we uncover how signals are transmitted across synapses to turn on the switch for plasticity,” Watanabe says. “We demonstrate that glutamate is first released near AMPA-type glutamate receptors, to relay the signal from one neuron to the next, and then near NMDA-type receptors immediately after the first signal to activate the switch for synaptic plasticity.”
This new study was also partly conducted in the MBL Neurobiology course, where Watanabe is a faculty member. “It began in 2018 with (course students) Raul Ramos and Hanieh Falahati, and then we followed up in 2019 with Stephen Alexander Lee and Christine Prater. Shuo Li, the first author, was my teaching assistant for the Neurobiology course for both years,” Watanabe says. He will be returning this summer to teach in the course --- and discover more!
Citation:
Shuo Li et al. (2021) Asynchronous release sites align with NMDA receptors in mouse hippocampal synapses. Nature Communications, DOI: 10.1038/s41467-021-21004-x.
